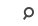A Randomized Double-Blind Parallel-Group Placebo Controlled Clinical Trial of Cordyceps Militaris M2-116-04 for Its Potential Sport and Exercise Nutrition Applications in Healthy Active Adults
LU IRB#: IRB-25-58
PI: Chad Kerksick, PhD
Description of Study
The aim of this study is to examine the effects of supplementation with Cordyceps militaris MS-116-04 on exercise performance, endurance, cardiovascular fitness, gastrointestinal wellness, mood, and recovery compared to a placebo in healthy active adults.
Location
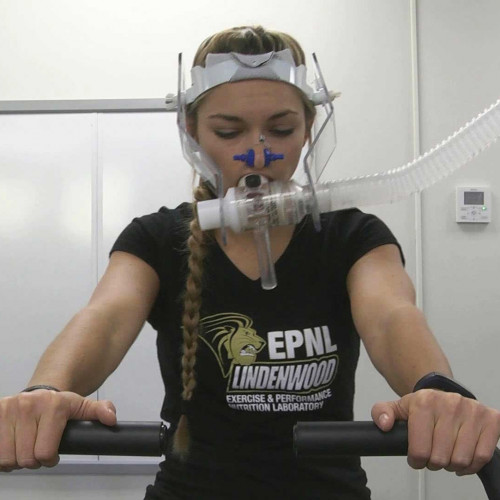
Exercise and Performance Nutrition Laboratory (Fieldhouse 126)
209 S Kingshighway St
Saint Charles, MO 63301Number of Visits
5Time Commitment
Visits 1: 1 hour
Visits 2: 1 hour
Visits 3: 5 hours
Visits 4: 1 hour
Visits 5: 5 hoursCompensation
$250Points of Contact
EPNL
epnl@lindenwood.edu
Research Sample Needed
- 36 healthy, active men and women
Inclusion Criteria
- Healthy males and females aged 21 to 45 years of age.
- Individual indicates they are currently engaged in and historically engaged in exercise (i.e., structured or unstructured) for the prior 12 months.
- Subject has the ability to exercise on a cycle ergometer without issue or concern.
- Subject has the ability to “sprint” (cycle Time to Exhaustion Trial) without any orthopedic or other limitations.
- Body Mass Index (BMI) 19 to 29.9 kg/m2 (normal weight to overweight status)
- Subject is in good health and appropriate for exercise as determined by physical examination, medical history and the Physical Activity Readiness Questionnaire (PAR-Q).
- Subject can exercise on a cycle ergometer without issue or concern.
- Subject is a non-smoker.
- Subject agrees to not use any new vitamin, mineral, or dietary supplement product until after study completion and to not take any vitamins, minerals or dietary supplements 24 hours prior to the exercise test visits.
- Subject is willing and able to comply with the protocol including: attending the study scheduled appointments, of which two of the study visits may take up to five hours for completion.
- Subject agrees to refrain from exercise for the 24 hours prior to any test visits.
- Subject agrees to refrain from alcohol use for at least 24 hours before every study visit.
- Subject agrees to refrain from using any antihistamines for the 24 hours prior to the exercise test visits.
- Subject agrees to refrain from drinking any exercise recovery beverages during the study period.
Exclusion Criteria
- Subject has any of the following medical conditions: active heart disease, uncontrolled high blood pressure (≥ 140/90 mmHg), renal or hepatic impairment/disease, Type I or II diabetes, bipolar disorder, Parkinson’s disease, unstable thyroid disease, immune disorder (such as HIV/AIDS), any medical condition deemed exclusionary by the Principal Investigator (PI)
- Subject has a history of cancer (except localized skin cancer without metastases) within 5 years prior to screening.
- Subject has a medical condition or orthopedic problem making exercising on a cycle ergometer contraindicated.
- Subject has a VO2 peak/max value above the following age/sex categorizations (as measured during Visit 1): Males 20-29y - 46.8 mL/kg/min, Males 30-39y - 45.3 mL/kg/min, Males 40-45y - 43.9 mL/kg/min, Females 20-29y - 40.6 mL/kg/min, females 30-39y - 38.1 mL/kg/min, females 40-45y - 35.6 mL/kg/min
- Subject is currently taking antihypertensives, hypoglycemic medications or stimulatory asthma medications.
- Subject is on an unstable dose of medication (defined as fewer than 90 days at the same dose).
- Subject is taking a prescription medication deemed exclusionary by the Principal Investigator (PI).
- Subject has an allergy to any ingredients in the Study Product.
- Subject has a history of drug or alcohol abuse in the past 12 months.
- Subject has a history of a psychiatric illness requiring hospitalization in the past 12 months.
- Subject has any condition or abnormality that in the expert opinion of the PI, participation in the study would compromise the safety of the subject or the quality of the study data.
Are You Eligible?
Information provided here reflects current IRB approval for this research. However, this information may be subject to change and updated accordingly.