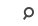Impact of Supplementing with Geranylgeraniol on Muscular Strength and Endurance, Body Composition, and Aerobic Capacity
LU IRB#: IRB-25-35
PI: Chad Kerksick, PhD
Description of Study
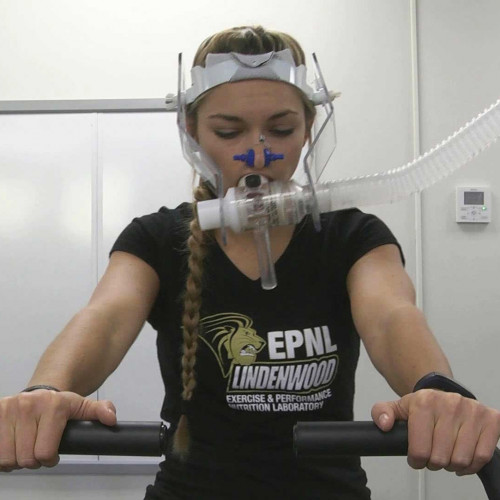
The aim of the research study is to examine the impact of supplementing with Geranylgeraniol (GG) in healthy, physically active men and women on changes in muscular strength and endurance, body composition, and aerobic capacity.
Location
Exercise and Performance Nutrition Laboratory (Fieldhouse 126)
209 S Kingshighway St
Saint Charles, MO 63301Number of Visits
4Time Commitment
Visits 1: 30-45 minutes
Visits 2-4: 60-120 minutesCompensation
$300Points of Contact
Alex Schrautemeier
epnl@lindenwood.edu
Research Sample Needed
- 60 healthy, physically active men and women
Inclusion Criteria
- Between 30 and 60 years old.
- Body mass index values will range from >18.5 and <29.9 kg/m2
- Subject agrees to maintain their existing dietary patterns throughout the study period.
- Subject agrees to refrain from alcohol, caffeine, and strenuous exercise for 24 hours prior to each test day.
- Is physically active, defined as at least 30 min of moderate exercise on at least 4 days a week.
- Avoid consumption of pomegranate juice and avoid consumption of CoQ10, vitamin B3 and its precursors, L-carnitine, MK-4
- Subject is willing and able to comply with the study protocol.
- Subject has given voluntary, written, informed consent to participate in the study.
Exclusion Criteria
- BMI <18.5 or > 29.9 kg/m2
- Positive medical history and/or is currently being treated for some form of heart disease, cardiovascular disease, kidney disease, renal failure, or has dialysis performed on regular intervals, Type I or Type II diabetes (determined as fasting blood glucose > 126 mg/dL), thyroid disease, liver disease or some form of clinically diagnosed hepatic impairment, immune disorder (i.e., HIV/AIDS), or neurological condition or disease.
- Diagnosed with any affective disorder or other psychiatric disorder that required hospitalization in the prior year.
- History of cancer (except localized skin cancer without metastases or in situ cervical cancer within 5 years prior to screening visit).
- Participant has an abnormality or obstruction of the gastrointestinal tract precluding swallowing (e.g., dysphagia) and digestion (e.g., known intestinal malabsorption, celiac disease, inflammatory bowel disease, chronic pancreatitis, steatorrhea)
- Genetic musculoskeletal and neurologic disorder known to affect skeletal muscle metabolism
- Has donated blood in past 60 days
- Diagnosed with or being treated for any endocrinological disorder and/or taking hormone boosting supplements (e.g. herbs) or hormone replacement therapy (prescribed/doctor ordered or not)
- Had CoQ10 supplement in past 30 days.
- Had steroid medication one month before starting the study
- Currently prescribed for the first time a statin drugs (i.e., Lipitor, Livalo, Crestor, Zocor, etc.) and/or a hypertension medication (i.e., Beta-blockers, ACE Inhibitors, Alpha blockers, Vasodilators, etc.) within the past 6 months or has had their dosage or medication changed within the past 6 months
- Current smoker (average of > 1 pack per week within the past 3 months) or has quit within the past six months. This includes all forms of nicotine
- Intake of any drugs (prescribed or over the counter) or dietary supplements that are known or are purported to impact energy expenditure or weight loss (caffeine doses <300 mg/day is permissible)
- Women with a history of hormone-related conditions such as endometriosis, fibroids, polycystic ovary syndrome
- Women who are pregnant, planning to become pregnant, or lactating currently or within the past six months
- Women who are currently using oral contraceptives. Those taking short-acting contraceptives (oral contraceptive pills) must have stopped taking them more than 3 months ago while those reporting any use of longer-acting hormonal contraceptives (e.g., Norplant (Wyeth-Ayerst), Depo-Provera (Pfizer), or hormonal intrauterine device) must have stopped taking them at least 12 months prior to beginning the study.
- Have a known sensitivity or allergy to any of the study products
- History of alcohol or substance abuse in the 12 months prior to screening
- Receipt or use of an investigational product in another research study within 30 days of beginning the study protocol
- Has participated in other clinical trials focused on physical and muscle performance within the last year
- They plan major changes in lifestyle (i.e., diet, dieting, exercise level, travel, etc.) during the study
- Individuals who regularly compete as part of sanctioned athletic activities or those individuals who regularly train more than 360 minutes of exercise per week
- Recent history (<3 months) of exercise training or weight loss (> 5%)
- Any condition or abnormality that, in the opinion of the investigator, would compromise the safety of the participant or the quality of the study data
Are You Eligible?
Information provided here reflects current IRB approval for this research. However, this information may be subject to change and updated accordingly.